Advertisement |
 |
Nature Catalysis is a new online-only journal that will publish work on all areas of catalysis. The journal will provide coverage of the science and business of catalysis research, creating a unique journal for scientists, engineers, and researchers in industry. Nature Catalysis is launching in January 2018 and is now open for submissions.
SUBMIT YOUR RESEARCH TODAY |  | |
 |
 |
TABLE OF CONTENTS
|
June 2017 Volume 9, Issue 6 |
 |  |  |
 |  Thesis Thesis
 News and Views News and Views
 Articles Articles
 In Your Element In Your Element | |
 |
|
 |
 |
Thesis |  Top Top |
 |
 |
 |
It figures pp501 - 502
Michelle Francl
doi:10.1038/nchem.2788
Using a slide rule showed Michelle Francl where chemists' calculations are still stuck in the past. |
 |
News and Views |  Top Top |
 |
 |
 |
|
 |
| Advertisement |
 |
npj Clean Water: open for submissions
An open access, online-only journal, dedicated to publishing high-quality papers that describe the significant and cutting-edge research that continues to ensure the supply of clean water to populations.
Explore the benefits of submitting your next manuscript. |  | |
 |
| |
Articles |  Top Top |
 |
 |
 |
Programming molecular self-assembly of intrinsically disordered proteins containing sequences of low complexity pp509 - 515
Joseph R. Simon, Nick J. Carroll, Michael Rubinstein, Ashutosh Chilkoti and Gabriel P. López
doi:10.1038/nchem.2715
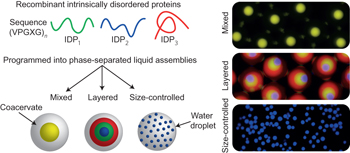
A programmable model of membraneless organelles comprised of intrinsically disordered proteins (IDPs) containing sequences of low complexity has now been developed. The rules governing the assembly of archetypal IDPs into biologically inspired mixed, layered and size-controlled configurations provides a new means for understanding intracellular phase behaviour of IDPs. |
 |
 |
 |
Coherent ultrafast lattice-directed reaction dynamics of triiodide anion photodissociation pp516 - 522
Rui Xian, Gastón Corthey, David M. Rogers, Carole A. Morrison, Valentyn I. Prokhorenko et al.
doi:10.1038/nchem.2751
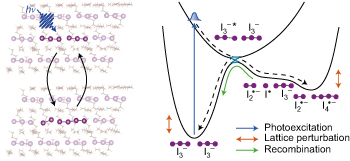
Dissociative reactions in the solid state are prone to sample damage. Now, improved sample handling and measurement conditions enable the study of the dissociative reaction of a model triatomic system in the solid state on ultrafast timescales, revealing the significant impact of lattice coordination on the reaction pathway.
See also: News and Views by Cerullo & Garavelli |
 |
 |
 |
Reactions of hexadehydro-Diels–Alder benzynes with structurally complex multifunctional natural products pp523 - 530
Sean P. Ross and Thomas R. Hoye
doi:10.1038/nchem.2732
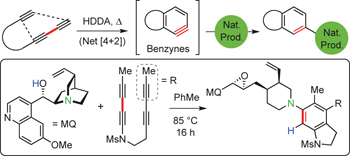
The development of methods for the site-selective modification of natural products is a topic of contemporary investigation and importance. Now, it has been shown that benzynes produced by the hexadehydro-Diels–Alder (HDDA) reaction react with many secondary metabolites, with a substantial preference for one of several potential pathways.
Chemical compounds
See also: News and Views by Tasker & Hergenrother |
 |
 |
 |
Monitoring interconversion between stereochemical states in single chirality-transfer complexes on a platinum surface pp531 - 536
Guillaume Goubert, Yi Dong, Michael N. Groves, J.-C. Lemay, Bjørk Hammer et al.
doi:10.1038/nchem.2753

A chiral molecule on a metal surface can set up a prochiral molecule for an enantioselective reaction step by forming a hydrogen-bonded complex that imposes a specific adsorption geometry. Time-lapsed scanning tunnelling microscopy and density functional theory studies reveal that such complexes can sometimes switch between states of opposing prochirality.
See also: News and Views by Tysoe |
 |
 |
 |
Engineering live cell surfaces with functional polymers via cytocompatible controlled radical polymerization pp537 - 545
Jia Niu, David J. Lunn, Anusha Pusuluri, Justin I. Yoo, Michelle A. O'Malley et al.
doi:10.1038/nchem.2713
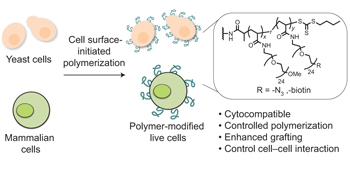
A cytocompatible controlled radical polymerization method has now been developed that initiates polymer synthesis directly on the surface of living cells. This method achieves significantly enhanced polymer grafting and enables active manipulation of cellular phenotypes. |
 |
 |
 |
Asymmetric silver-catalysed intermolecular bromotrifluoromethoxylation of alkenes with a new trifluoromethoxylation reagent pp546 - 551
Shuo Guo, Fei Cong, Rui Guo, Liang Wang and Pingping Tang
doi:10.1038/nchem.2711
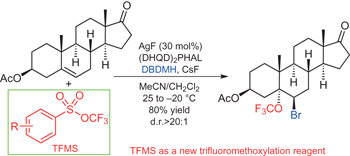
The first example of an asymmetric silver-catalysed intermolecular bromotrifluoromethoxylation of alkenes has been described with trifluoromethyl aryl sulfonate as a new trifluoromethoxylation reagent. This reaction is operationally simple, scalable and proceeds under mild reaction conditions, which can be applied to the late-stage trifluoromethoxylation of complex small molecules.
Chemical compounds |
 |
 |
 |
Structural and functional synthetic model of mono-iron hydrogenase featuring an anthracene scaffold pp552 - 557
Junhyeok Seo, Taylor A. Manes and Michael J. Rose
doi:10.1038/nchem.2707
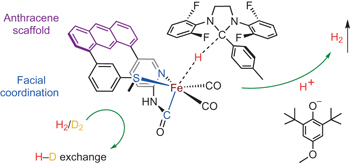
Mono-iron hydrogenase promotes the heterolytic cleavage of H2 and subsequent hydride transfer to its organic substrate, H4MPT+, which serves as a CO2 ‘carrier’ in methanogenic pathways. Now, using an anthracene-scaffold-based approach, a synthetic model featuring enzyme-like Fe-C,N,S facial coordination has been developed. The model complex enables the bidirectional activity of H2 activation and evolution.
Chemical compounds |
 |
 |
 |
Catalytic enantioselective synthesis of atropisomeric biaryls by a cation-directed O-alkylation pp558 - 562
John D. Jolliffe, Roly J. Armstrong and Martin D. Smith
doi:10.1038/nchem.2710
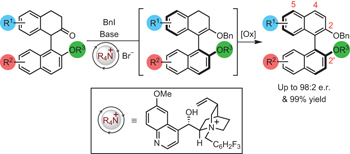
A chiral ammonium salt mediates a dynamic kinetic resolution of racemic α-aryl ketones by atropselective O-alkylation. Oxidation with DDQ gives access to C2-symmetric and non-symmetric BINOL derivatives in high yields and with high enantioselectivity.
Chemical compounds |
 |
 |
 |
A two-dimensional conjugated aromatic polymer via C–C coupling reaction pp563 - 570
Wei Liu, Xin Luo, Yang Bao, Yan Peng Liu, Guo-Hong Ning et al.
doi:10.1038/nchem.2696
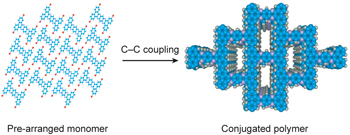
The synthesis of well-defined planar polymers presents a significant challenge for chemists seeking to investigate their potential for use in emerging technologies. Now, a two-dimensional conjugated aromatic polymer has been synthesized via endogenous solid-state polymerization of pre-arranged monomers, and its performance as an organic anode in an ambient temperature sodium cell tested. |
 |
 |
 |
Unique physicochemical and catalytic properties dictated by the B3NO2 ring system pp571 - 577
Hidetoshi Noda, Makoto Furutachi, Yasuko Asada, Masakatsu Shibasaki and Naoya Kumagai
doi:10.1038/nchem.2708
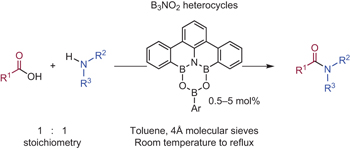
Amidation is one of the most widely utilized organic reactions for the synthesis of pharmaceuticals and functional materials. DATB, characterized by the B3NO2 heterocycle, proved to act as a superb catalyst for the direct amidation with a distinct mechanistic pathway, displaying broadened applicability to a wide range of substrates.
Chemical compounds |
 |
 |
 |
Actinide covalency measured by pulsed electron paramagnetic resonance spectroscopy pp578 - 583
Alasdair Formanuik, Ana-Maria Ariciu, Fabrizio Ortu, Reece Beekmeyer, Andrew Kerridge et al.
doi:10.1038/nchem.2692
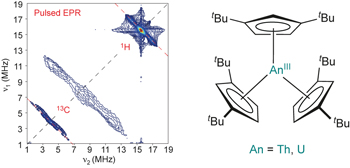
Covalency in actinide–ligand bonding is poorly understood compared to that in other parts of the periodic table due to the lack of experimental data. Here, pulsed electron paramagnetic resonance methods are used to directly measure the electron spin densities at coordinated ligands in molecular thorium and uranium complexes. |
 |
 |
 |
Prebiotic selection and assembly of proteinogenic amino acids and natural nucleotides from complex mixtures pp584 - 589
Saidul Islam, Dejan-Krešimir Bucar and Matthew W. Powner
doi:10.1038/nchem.2703
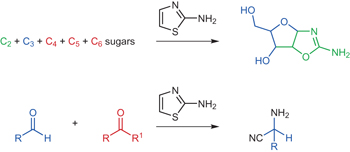
2-aminothiazole — a hybrid of prebiotic amino acid and nucleotide precursors — sequentially accumulates and purifies glycolaldehyde and glyceraldehyde from complex mixtures in the order required for ribonucleotide synthesis, dynamically resolves glyceraldehyde from its ketose-isomer dihydroxyacetone, and provides the first strategy to select natural amino acids from abiotic aldehydes and ketones.
Chemical compounds |
 |
 |
 |
Switchable regioselectivity in amine-catalysed asymmetric cycloadditions pp590 - 594
Zhi Zhou, Zhou-Xiang Wang, Yuan-Chun Zhou, Wei Xiao, Qin Ouyang et al.
doi:10.1038/nchem.2698
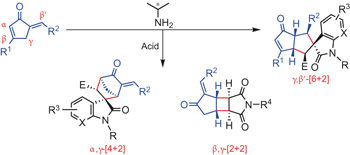
The construction of diversified compound libraries from identical substrates is attractive but remains a challenge in asymmetric synthesis. Here, we demonstrate switchable regioselective [6+2], [4+2] or [2+2] cycloadditions with α′-alkylidene-2-cyclopentenones via mild aminocatalysis, producing a spectrum of chiral frameworks with high structural diversity and molecular complexity.
Chemical compounds |
 |
 |
 |
Activation and discovery of earth-abundant metal catalysts using sodium tert-butoxide pp595 - 600
Jamie H. Docherty, Jingying Peng, Andrew P. Dominey and Stephen P. Thomas
doi:10.1038/nchem.2697
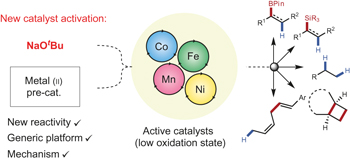
NaOtBu — an alkoxide salt — enables simple access to low-oxidation-state catalysis using sustainable first-row transition metals (Fe, Co, Mn, Ni). The approach works across a wide range of reductive alkene and alkyne functionlization reactions including hydroboration, hydrosilylation, hydrogenation, hydrovinylation and [2π+2π] cyclization reactions.
Chemical compounds |
 |
| Advertisement |
 |
An open access, online-only, multidisciplinary research journal dedicated to publishing the most important scientific advances in the life sciences, physical sciences, and engineering fields that are facilitated by spaceflight and analogue platforms.
Explore the benefits of submitting your next research article. |  | |
 |
| |
In Your Element |  Top Top |
 |
 |
 |
V for vanadium p602
Andrea Taroni
doi:10.1038/nchem.2787
Andrea Taroni shares his experience with vanadium — a colourful element with a rich chemistry (and physics!) that is emblematic of all transition metals. |
 |
 Top Top |
 |
 |
 |  |  |  |  |  | Natureevents is a fully searchable, multi-disciplinary database designed to maximise exposure for events organisers. The contents of the Natureevents Directory are now live. The digital version is available here.
Find the latest scientific conferences, courses, meetings and symposia on natureevents.com. For event advertising opportunities across the Nature Publishing Group portfolio please contact natureevents@nature.com |  |  |  |  |  |
|
 |


No comments:
Post a Comment