TABLE OF CONTENTS
|
January 2017 Volume 9, Issue 1 |
 |  |  |
 |  Commentary Commentary
 Thesis Thesis
 News and Views News and Views
 Perspective Perspective
 Articles Articles
 In Your Element In Your Element | |
 |
|
 |
 |
Commentary |  Top Top |
 |
 |
 |
Bridging the gaps in 18F PET tracer development pp1 - 3
Michael G. Campbell, Joel Mercier, Christophe Genicot, Veronique Gouverneur, Jacob M. Hooker et al.
doi:10.1038/nchem.2693
As compared to the drug discovery process, the development of new 18F PET tracers lacks a well-established pipeline that advances compounds from academic research to candidacy for (pre)clinical imaging. In order to bridge the gaps between methodological advances and clinical success, we must rethink the development process from training to implementation. |
 |
Thesis |  Top Top |
 |
 |
 |
Chemists boldly go pp4 - 5
Michelle Francl and Michael Donnay
doi:10.1038/nchem.2702
Michael Donnay and Michelle Francl want chemists to share the stories behind the work they do, and not be afraid to identify the heroines and heroes — and their epic adventures — that paved the way. |
 |
News and Views |  Top Top |
 |
 |
 |
|
 |
Perspective |  Top Top |
 |
 |
 |
Interplay between defects, disorder and flexibility in metal-organic frameworks pp11 - 16
Thomas D. Bennett, Anthony K. Cheetham, Alain H. Fuchs and François-Xavier Coudert
doi:10.1038/nchem.2691
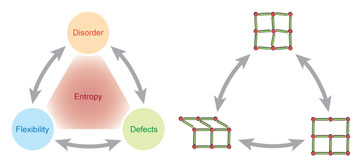
Although metal-organic frameworks are often seen as rigid crystalline structures, there is growing evidence that large-scale flexibility, the presence of defects, and long-range disorder are not the exception, but rather the norm. Here we propose that these concepts are inescapably intertwined, and the interfaces between them offer prospects for enhancement of materials' functionalities. |
 |
Articles |  Top Top |
 |
 |
 |
Reticular synthesis of porous molecular 1D nanotubes and 3D networks pp17 - 25
A. G. Slater, M. A. Little, A. Pulido, S. Y. Chong, D. Holden et al.
doi:10.1038/nchem.2663
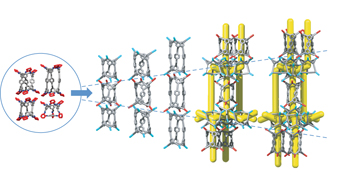
Porous molecular crystals have desirable properties, but are hard to form with the level of structural control seen for extended framework materials. Now, a ‘mix-and-match’ chiral recognition strategy has been used to form reticular porous supramolecular nanotubes and 3D networks, providing a blueprint for pore design in molecular crystals.
See also: News and Views by Mellot-Draznieks & Cheetham |
 |
 |
 |
Site-selective C–H arylation of primary aliphatic amines enabled by a catalytic transient directing group pp26 - 32
Yongbing Liu and Haibo Ge
doi:10.1038/nchem.2606
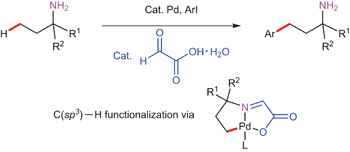
Transition-metal-catalysed direct C(sp3)−H functionalization of primary aliphatic amines is an attractive — but elusive — process that could provide efficient access to biologically and pharmaceutically important compounds. Now, a palladium-catalysed γ-arylation of primary alkylamines is achieved using an inexpensive, catalytic and transient directing group.
Chemical compounds |
 |
 |
 |
Fusing tetrapyrroles to graphene edges by surface-assisted covalent coupling pp33 - 38
Yuanqin He, Manuela Garnica, Felix Bischoff, Jacob Ducke, Marie-Laure Bocquet et al.
doi:10.1038/nchem.2600
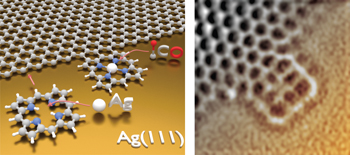
Lateral anchoring of heteromolecules to graphene paves the way for the creation of hybrid materials with tunable properties. Now, following a surface-assisted dehydrogenative coupling reaction, the edges of graphene on silver have been functionalized with porphines. This enables the assembly of well-defined multifunctional graphene-based nanostructures. |
 |
 |
 |
An infrared spectroscopy approach to follow β-sheet formation in peptide amyloid assemblies pp39 - 44
Jongcheol Seo, Waldemar Hoffmann, Stephan Warnke, Xing Huang, Sandy Gewinner et al.
doi:10.1038/nchem.2615
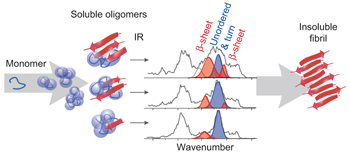
There is increasing evidence that highly dynamic, polydisperse peptide oligomers are the toxic species in amyloid-related diseases such as Alzheimer's and Parkinson's. Now, the secondary structure of individual amyloid oligomers has been analysed directly for the first time using a combination of ion-mobility spectrometry–mass spectrometry and gas-phase infrared spectroscopy. |
 |
 |
 |
Lack of evidence for phase-only control of retinal photoisomerization in the strict one-photon limit pp45 - 49
M. Liebel and P. Kukura
doi:10.1038/nchem.2598
The degree to which light-induced processes are sensitive to the shape of an incident electromagnetic wave remains a hotly debated topic. Experiments performed at very low levels of light agree with seminal theoretical predictions that tuning the phase of the light field does not affect photochemical reactivity at the single-photon level. |
 |
 |
 |
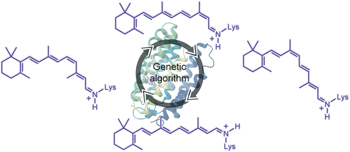
Emergence of a catalytic tetrad during evolution of a highly active artificial aldolase pp50 - 56
Richard Obexer, Alexei Godina, Xavier Garrabou, Peer R. E. Mittl, David Baker et al.
doi:10.1038/nchem.2596
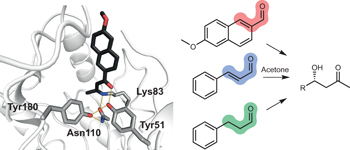
An artificial aldolase has been optimized using an ultrahigh-throughput microfluidic screening assay. The evolved enzyme exhibits excellent stereoselectivity and broad substrate scope. Structural studies suggest that a Lys-Tyr-Asn-Tyr catalytic tetrad, which emerged during directed evolution, is responsible for the >109 rate enhancement achieved by this catalyst. |
 |
 |
 |
Homochiral polymerization-driven selective growth of graphene nanoribbons pp57 - 63
Hiroshi Sakaguchi, Shaotang Song, Takahiro Kojima and Takahiro Nakae
doi:10.1038/nchem.2614
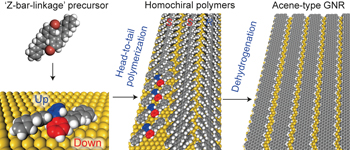
Metal surfaces have been believed to be catalytic, but the mechanism of catalysis is unknown. Now, graphene nanoribbons (GNRs) can be grown on Au(111) from a 'Z-bar-linkage' precursor through a conformation-controlled mechanism. Chemical vapour deposition of precursors adopting a chiral conformation produced homochiral polymers, which are dehydrogenated to form GNRs.
Chemical compounds |
 |
 |
 |
Breaking scaling relations to achieve low-temperature ammonia synthesis through LiH-mediated nitrogen transfer and hydrogenation pp64 - 70
Peikun Wang, Fei Chang, Wenbo Gao, Jianping Guo, Guotao Wu et al.
doi:10.1038/nchem.2595
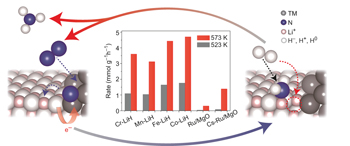
The existence of linear scaling relations between the adsorption energies of reaction intermediates on transition-metal surfaces prevents their independent optimization and limits catalytic activity. It has now been shown that using a catalytic LiH site alongside a transition-metal catalyst can break these intrinsic scaling relations, leading to unprecedented lower-temperature ammonia-synthesis activity. |
 |
 |
 |
Conformer-specific hydrogen atom tunnelling in trifluoromethylhydroxycarbene pp71 - 76
Artur Mardyukov, Henrik Quanz and Peter R. Schreiner
doi:10.1038/nchem.2609
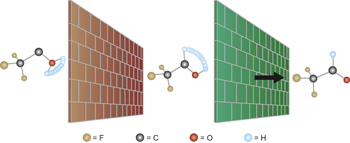
Control over the selectivity of chemical reactions and biological molecules requires an intimate understanding of how conformation impacts reactivity. It is now shown that the trans conformer of trifluoromethylhydroxycarbene preferentially rearranges through a quantum-mechanical hydrogen-tunnelling pathway, whereas its cis conformer is unreactive. |
 |
 |
 |
Multistep nucleation of nanocrystals in aqueous solution pp77 - 82
N. Duane Loh, Soumyo Sen, Michel Bosman, Shu Fen Tan, Jun Zhong et al.
doi:10.1038/nchem.2618
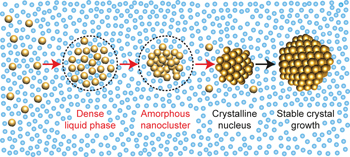
Crystals grow from nuclei. In systems where nuclei are nanometre-sized and form quickly, it is difficult to determine the mechanism of their formation. Now, through in situ TEM, the demixing of a supersaturated aqueous gold solution into metastable gold-poor and gold-rich liquid phases is observed, the latter yielding stable clusters that become nuclei for nanocrystal growth. |
 |
 |
 |
Suppression of Kasha's rule as a mechanism for fluorescent molecular rotors and aggregation-induced emission pp83 - 87
Hai Qian, Morgan E. Cousins, Erik H. Horak, Audrey Wakefield, Matthew D. Liptak et al.
doi:10.1038/nchem.2612

A family of fluorescent molecular rotors has been developed and their mechanism for emission understood. It has been observed that, although most fluorescent molecules emit from their lowest energy excited state, S1 (in accordance with Kasha's rule), BODIHY dyes do not. Furthermore, their fluorescence is enhanced through restricted rotor rotation, which suppresses internal conversion to the dark S1 state.
Chemical compounds |
 |
 |
 |
Mechanism of O2 diffusion and reduction in FeFe hydrogenases pp88 - 95
Adam Kubas, Christophe Orain, David De Sancho, Laure Saujet, Matteo Sensi et al.
doi:10.1038/nchem.2592
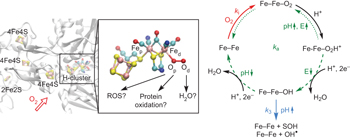
FeFe hydrogenases are highly efficient H2 producing enzymes; however, they can be inactivated by O2. Now, a mechanism for O2 diffusion within FeFe hydrogenases and its reactions at the active site of the enzyme has been proposed. These findings could help with the design of hydrogenase mutants with increased resistance to oxidative damage. |
 |
In Your Element |  Top Top |
 |
 |
 |
A portrait of cadmium p96
Nadezda V. Tarakina and Bart Verberck
doi:10.1038/nchem.2699
Nadezda V. Tarakina and Bart Verberck explore the colourful history and assets of element 48. |
 |
 Top Top |
 |
 |
 |  |  |  |  |  | Natureevents is a fully searchable, multi-disciplinary database designed to maximise exposure for events organisers. The contents of the Natureevents Directory are now live. The digital version is available here.
Find the latest scientific conferences, courses, meetings and symposia on natureevents.com. For event advertising opportunities across the Nature Publishing Group portfolio please contact natureevents@nature.com |  |  |  |  |  |
|
 |


No comments:
Post a Comment