Advertisement |
 |
Nature Awards for Mentoring in Science — Spain
Your mentor could win €10,000
Nominations are now open | | | |
 |
 |
TABLE OF CONTENTS
|
July 2017 Volume 13, Issue 7 |
 |  |  |
 |  Research Highlights Research Highlights
 News and Views News and Views
 Perspective Perspective
 Brief Communication Brief Communication
 Articles Articles
| |
 |
|
 |
 |
Advertisement |
 |
| |
 |
| |
| Advertisement |
 |
Naturejobs Career Guide Melbourne 2017
Melbourne's institutes cluster into discrete geographic hubs, with a solid culture of collaboration and an emphasis on biomedical research. Learn more about the area's leading scientific entities; their ties to each other; success from a scientific data-driven perspective and much more.
Read the full Career Guide |  | | |
 |
| |
Research Highlights |  Top Top |
 |
 |
 |
Metal homeostasis: Pumping iron | Antimicrobials: Targeting fungal chromatin | Synthetic biology: Playing favorites | Circadian regulation: Switching periods
|
News and Views |  Top Top |
 |
 |
 |
| |
 |
Perspective |  Top Top |
 |
 |
 |
|
 |
Brief Communication |  Top Top |
 |
 |
 |
Engineering RGB color vision into Escherichia coli pp706 - 708
Jesus Fernandez-Rodriguez, Felix Moser, Miryoung Song and Christopher A Voigt
doi:10.1038/nchembio.2390
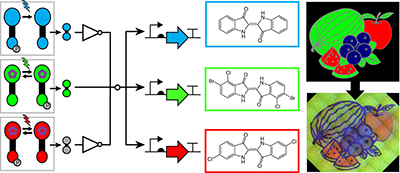
A synthetic biology system composed of light-wavelength-responsive genetic regulators, signal-processing circuits and pigment-production pathways have resulted in an Escherichia coli strain that can record color images in RGB format.
|
|
|
 |
Articles |  Top Top |
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
Membrane curvature regulates ligand-specific membrane sorting of GPCRs in living cells pp724 - 729
Kadla R Rosholm, Natascha Leijnse, Anna Mantsiou, Vadym Tkach, Soren L Pedersen et al.
doi:10.1038/nchembio.2372
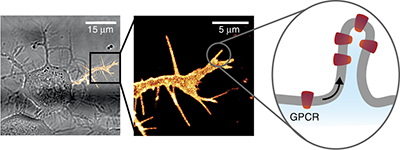
Membrane curvature induces sorting of GPCRs within live-cell-membrane protrusions, and the curvature-dependent sorting is affected by agonist binding. Thermodynamic modeling suggests that this is due to an energetic drive to match receptor shape and elasticity to membrane curvature.
|
|
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
Functional selectivity of GPCR-directed drug action through location bias pp799 - 806
Roshanak Irannejad, Veronica Pessino, Delphine Mika, Bo Huang, Philip B Wedegaertner et al.
doi:10.1038/nchembio.2389
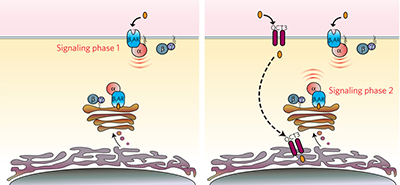
Intracellular Gs-cAMP signaling from β1-adrenergic receptors residing in the Golgi represents /`location bias/', a new form of GPCR functional selectivity. Inactive Golgi-localized receptors can be activated by some β1-targeting drugs.
|
|
|
 |
 |
 |
Structural basis for high-affinity fluorophore binding and activation by RNA Mango pp807 - 813
Robert J Trachman III, Natalia A Demeshkina, Matthew W L Lau, Shanker Shyam S Panchapakesan, Sunny C Y Jeng et al.
doi:10.1038/nchembio.2392
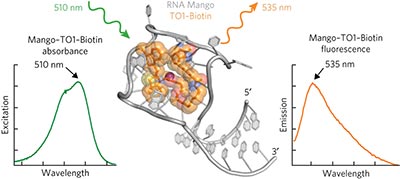
A crystal structure of the RNA aptamer Mango bound to a thiazole orange-derived fluorophore reveals a three-tiered G-quadruplex structure, which, together with three flap-like nucleotides, constrains the fluorophore into its active conformation.
|
|
|
 |
 |  |  |  |  |  | Natureevents is a fully searchable, multi-disciplinary database designed to maximise exposure for events organisers. The contents of the Natureevents Directory are now live. The digital version is available here.
Find the latest scientific conferences, courses, meetings and symposia on natureevents.com. For event advertising opportunities across the Nature Publishing Group portfolio please contact natureevents@nature.com |  |  |  |  |  |
|
 |


No comments:
Post a Comment