Advertisement |
 |
Nature Reviews Chemistry is an online-only journal that provides both an introduction to chemists embarking on a new topic of investigation and thought-provoking, in-depth sections for the expert. The journal also publishes regular columns which focus on the teaching of chemistry and on the translation of research into business opportunities.
EXPLORE THE JOURNAL NOW |  | | |
 |
 |
TABLE OF CONTENTS
|
September 2017 Volume 13, Issue 9 |
 |  |  |
 |  Research Highlights Research Highlights
 News and Views News and Views
 Perspective Perspective
 Articles Articles
| |
 |
|
 |
 |
| Advertisement |
 |
Nature Catalysis is a new online-only journal that will publish work on all areas of catalysis. The journal will provide coverage of the science and business of catalysis research, creating a unique journal for scientists, engineers, and researchers in industry. Nature Catalysis is launching in January 2018 and is now open for submissions.
SUBMIT YOUR RESEARCH TODAY |  | | |
 |
| |
Research Highlights |  Top Top |
 |
 |
 |
Ubiquitin-proteasome system: Rescuing EBV latency | Protein design: I like to fold it, fold it | Bacterial immunity: A virtuous CRISPR cycle | Neurobiology: Lighting up neurons
|
News and Views |  Top Top |
 |
 |
 |
| |
 |
Perspective |  Top Top |
 |
 |
 |
A kinetic view of GPCR allostery and biased agonism pp929 - 937
J Robert Lane, Lauren T May, Robert G Parton, Patrick M Sexton and Arthur Christopoulos
doi:10.1038/nchembio.2431
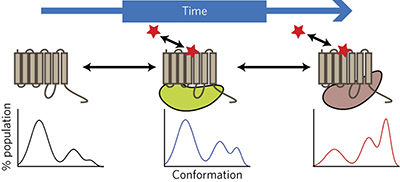
Allosteric modulation and biased agonism at GPCRs could be manifestations of the same underlying 'conformational selection' mechanism, and these can be harmonized by considering the influence of ligand-receptor residence time and kinetic context.
|
|
|
 |
Articles |  Top Top |
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
Allosteric sensitization of proapoptotic BAX pp961 - 967
Jonathan R Pritz, Franziska Wachter, Susan Lee, James Luccarelli, Thomas E Wales et al.
doi:10.1038/nchembio.2433
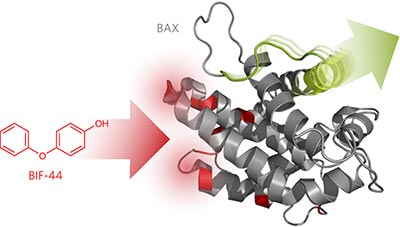
An NMR fragment screen identified a small molecule that binds to an allosteric site on the proapoptotic protein BAX and synergizes with the BIM BH3 domain to conformationally activate BAX and enhance BAX-mediated membrane poration.
Chemical compounds
|
|
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
FRET monitoring of a nonribosomal peptide synthetase pp1009 - 1015
Jonas Alfermann, Xun Sun, Florian Mayerthaler, Thomas E Morrell, Eva Dehling et al.
doi:10.1038/nchembio.2435
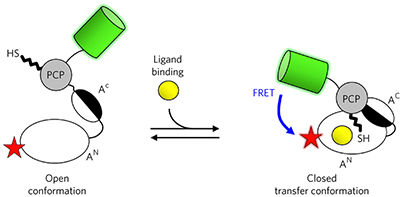
FRET sensors based on the adenylation and peptidyl carrier protein domains of a nonribosomal peptide synthetase illuminate the relationships between conformational dynamics and the catalytic cycle of this multidomain assembly line enzyme.
|
|
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |  |  |  |  |  | Natureevents is a fully searchable, multi-disciplinary database designed to maximise exposure for events organisers. The contents of the Natureevents Directory are now live. The digital version is available here.
Find the latest scientific conferences, courses, meetings and symposia on natureevents.com. For event advertising opportunities across the Nature Publishing Group portfolio please contact natureevents@nature.com |  |  |  |  |  |
|
 |


No comments:
Post a Comment