Advertisement |
 |
| |
 |
 |
TABLE OF CONTENTS
|
August 2017 Volume 13, Issue 8 |
 |  |  |
 |  Editorial Editorial
 Research Highlights Research Highlights
 News and Views News and Views
 Perspective Perspective
 Brief Communications Brief Communications
 Articles Articles
 Errata Errata
|  | Advertisement |  |  |  |  Nature Reviews Chemistry is an online-only journal that provides both an introduction to chemists embarking on a new topic of investigation and thought-provoking, in-depth sections for the expert. The journal also publishes regular columns which focus on the teaching of chemistry and on the translation of research into business opportunities. Nature Reviews Chemistry is an online-only journal that provides both an introduction to chemists embarking on a new topic of investigation and thought-provoking, in-depth sections for the expert. The journal also publishes regular columns which focus on the teaching of chemistry and on the translation of research into business opportunities.
EXPLORE THE JOURNAL NOW | | |
|
|
 |
|
 |
 |
| Advertisement |
 |
Nature Catalysis is a new online-only journal that will publish work on all areas of catalysis. The journal will provide coverage of the science and business of catalysis research, creating a unique journal for scientists, engineers, and researchers in industry. Nature Catalysis is launching in January 2018 and is now open for submissions.
SUBMIT YOUR RESEARCH TODAY |  | | |
 |
| |
Editorial |  Top Top |
 |
 |
 |
Hints of hidden chemistry p815
doi:10.1038/nchembio.2449
Biosynthetic pathways harbor diverse enzyme functions, and identifying those that catalyze unusual or synthetically challenging transformations offers new routes for biocatalytic development.
|
 |
Research Highlights |  Top Top |
 |
 |
 |
Neurodegenerative disease: Not stuck on repeat | Enzymology: Tracking off-targets | Antibacterials: PUMmeling RNAP | Biocatalysis: Achieving aminase activity
|
News and Views |  Top Top |
 |
 |
 |
| |
 |
Perspective |  Top Top |
 |
 |
 |
Harnessing yeast organelles for metabolic engineering pp823 - 832
Sarah K Hammer and José L Avalos
doi:10.1038/nchembio.2429
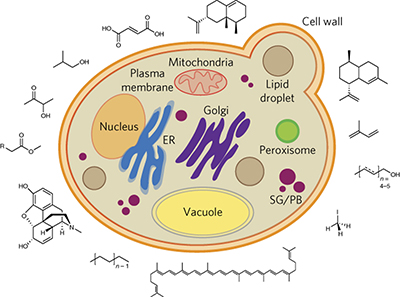
The organelles and subcellular compartments of yeast provide distinct environments and physical separation from the cytosol, enabling opportunities to target biosynthetic pathways to these compartments and enhance production of desirable compounds.
|
|
|
 |
Brief Communications |  Top Top |
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
Articles |  Top Top |
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
A mutant O-GlcNAcase enriches Drosophila developmental regulators pp882 - 887
Nithya Selvan, Ritchie Williamson, Daniel Mariappa, David G Campbell, Robert Gourlay et al.
doi:10.1038/nchembio.2404
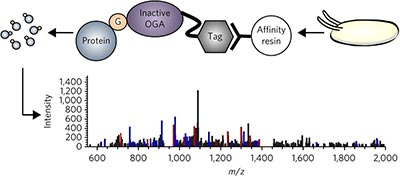
An inactive mutant of a bacterial O-GlcNAc hydrolase was used as an affinity reagent to enrich O-GlcNAc-modified proteins from Drosophila embryos and led to the identification, by MS-proteomics, of O-GlcNAcylated proteins involved in embryogenesis.
|
|
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
Errata |  Top Top |
 |
 |
 |
Erratum: Structural and conformational determinants of macrocycle cell permeability p922
Björn Over, Pär Matsson, Christian Tyrchan, Per Artursson, Bradley C Doak et al.
doi:10.1038/nchembio0817-922a
|
 |
 |
 |
Erratum: The EED protein–protein interaction inhibitor A-395 inactivates the PRC2 complex p922
Yupeng He, Sujatha Selvaraju, Michael L Curtin, Clarissa G Jakob, Haizhong Zhu et al.
doi:10.1038/nchembio0817-922b
|
 |
 Top Top |
 |
 |
 |  |  |  |  |  | Natureevents is a fully searchable, multi-disciplinary database designed to maximise exposure for events organisers. The contents of the Natureevents Directory are now live. The digital version is available here.
Find the latest scientific conferences, courses, meetings and symposia on natureevents.com. For event advertising opportunities across the Nature Publishing Group portfolio please contact natureevents@nature.com |  |  |  |  |  |
|
 |


No comments:
Post a Comment