TABLE OF CONTENTS
|
March 2017 Volume 13, Issue 3 |
 |  |  |
 |  Research Highlights Research Highlights
 News and Views News and Views
 Perspective Perspective
 Brief Communications Brief Communications
 Articles Articles
| |
 |
|
 |
 |
Research Highlights |  Top Top |
 |
 |
 |
Plant infection: A decoy tactic | mRNA localization: If you have to ASH | Enzyme mechanisms: Fickle about fluorine | Metabolism: A-way with biofilms
|
News and Views |  Top Top |
 |
 |
 |
| |
 |
Perspective |  Top Top |
 |
 |
 |
|
 |
Brief Communications |  Top Top |
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
MraY-antibiotic complex reveals details of tunicamycin mode of action pp265 - 267
Jonna K Hakulinen, Jenny Hering, Gisela Branden, Hongming Chen, Arjan Snijder et al.
doi:10.1038/nchembio.2270
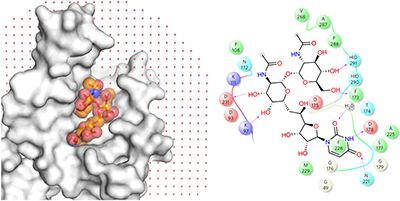
A structural study of MraY, an essential enzyme from Clostridium bolteae involved in bacterial cell wall synthesis, in complex with the natural product antibiotic tunicamycin, provides a basis for future antibiotic design.
|
|
|
 |
Articles |  Top Top |
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |
 |
|
 |
 |  |  |  |  |  | Natureevents is a fully searchable, multi-disciplinary database designed to maximise exposure for events organisers. The contents of the Natureevents Directory are now live. The digital version is available here.
Find the latest scientific conferences, courses, meetings and symposia on natureevents.com. For event advertising opportunities across the Nature Publishing Group portfolio please contact natureevents@nature.com |  |  |  |  |  |
|
 |


No comments:
Post a Comment