|
|
 |
TABLE OF CONTENTS
|
November 2015 Volume 7, Issue 11 |
 |  |  |
 |  Thesis Thesis
 News and Views News and Views
 Perspective Perspective
 Articles Articles
 In Your Element In Your Element | |
 |
|
 |
 |
| Advertisement |
 |
Nature Energy: Call for Papers
Launching in January 2016, Nature Energy is now open for submissions and inviting high-quality research from across the natural and social sciences. The journal will be dedicated to exploring all aspects of the on-going discussion of energy provision; from the generation and storage of energy, to its distribution and management, the needs and demands of the different actors, and the impacts that energy technologies and policies have on societies.
Submit your next research paper to the journal. | | | |
 |
| |
Advertisement |
 |
Nature Video Nobel laureates in their own words
“Are you sitting comfortably? Then let me tell you about my Nobel prize-winning science.”
In this series of animations, Nobel prize-winning scientists talk about work, life and discoveries that change the world. Recorded at the 65th Lindau Nobel Laureate Meeting.
Watch the animations free online.
Supported by Mars, Incorporated |  | | |
 |
| |
Thesis |  Top Top |
 |
 |
 |
Hard-luck Scheele pp855 - 856
Bruce C. Gibb
doi:10.1038/nchem.2379
Carl Wilhelm Scheele had a hand in the discovery of at least six elements and contributed to the early development of chemistry in numerous other ways. Bruce Gibb looks into Scheele's story and considers why he doesn't get the credit that he deserves. |
 |
News and Views |  Top Top |
 |
 |
 |
|
 |
Perspective |  Top Top |
 |
 |
 |
Palladium-catalysed norbornene-mediated C-H functionalization of arenes pp863 - 870
Juntao Ye and Mark Lautens
doi:10.1038/nchem.2372
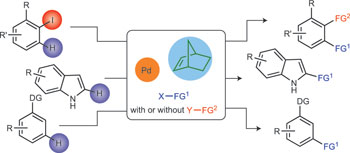
C-H functionalization of organic compounds is an ideal yet challenging approach to organic synthesis. This Perspective covers the most recent developments concerning the palladium-catalysed norbornene-mediated C-H functionalization of arenes, including applications of these methodologies in natural products synthesis. Challenges as well as opportunities for future studies are also presented. |
 |
Articles |  Top Top |
 |
 |
 |
Shaping quaternary assemblies of water-soluble non-peptide helical foldamers by sequence manipulation pp871 - 878
Gavin W. Collie, Karolina Pulka-Ziach, Caterina M. Lombardo, Juliette Fremaux, Frédéric Rosu et al.
doi:10.1038/nchem.2353

The self-assembly of short amphiphilic a-helicomimetic foldamers bearing proteinaceous side-chains can be controlled by manipulating the side-chain sequence. This enables the foldamers to be programmed to form either discrete helical bundles containing isolated cavities, or pH-responsive water-filled channels with controllable pore diameters.
See also: News and Views by Horne |
 |
 |
 |
An eight-step synthesis of epicolactone reveals its biosynthetic origin pp879 - 882
Pascal Ellerbrock, Nicolas Armanino, Marina K. Ilg, Robert Webster and Dirk Trauner
doi:10.1038/nchem.2336

Analysis of the structure of the highly complex yet racemic secondary metabolite epicolactone suggests that it may arise biosynthetically from a cascade similar to that which produces purpurogallin. This led to a synthesis of epicolactone in only eight steps using an intricate reaction cascade.
Chemical compounds
See also: News and Views by Mercer & Burns |
 |
 |
 |
Iron sensitizer converts light to electrons with 92% yield pp883 - 889
Tobias C. B. Harlang, Yizhu Liu, Olga Gordivska, Lisa A. Fredin, Carlito S. Ponseca, Jr et al.
doi:10.1038/nchem.2365

Using iron instead of the scarce ruthenium in light-harvesting complexes is challenging because iron complexes generally have short-lived excited states. Now an iron complex has been developed that has a long-lived excited state, which can lead to photo-induced electron injection into nanoporous TiO2 with a yield of 92%.
Chemical compounds
See also: News and Views by Galoppini |
 |
 |
 |
Unidirectional rotary motion in achiral molecular motors pp890 - 896
Jos C. M. Kistemaker, Peter Štacko, Johan Visser and Ben L. Feringa
doi:10.1038/nchem.2362

Avoiding equal probability for clockwise and anticlockwise rotation is essential for the function of molecular motors, and both biological and synthetic systems take advantage of chirality to control the rotary direction. Now it has been shown, by integrating two rotor moieties in a symmetric meso motor design, that light-driven unidirectional rotary motion can be achieved in an achiral system.
Chemical compounds |
 |
 |
 |
Co-assembly, spatiotemporal control and morphogenesis of a hybrid protein–peptide system pp897 - 904
Karla E. Inostroza-Brito, Estelle Collin, Orit Siton-Mendelson, Katherine H. Smith, Amàlia Monge-Marcet et al.
doi:10.1038/nchem.2349

Amphiphilic-peptide-driven opening of elastin-like protein molecules triggers the self-assembly of a multilayered membrane. This dynamic system can undergo morphogenesis into hierarchically ordered tubular structures that can be used to create complex scaffolds for tissue engineering. |
 |
 |
 |
Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts pp905 - 912
Hong Xu, Jia Gao and Donglin Jiang
doi:10.1038/nchem.2352

Covalent organic frameworks (COFs) feature periodic layers and ordered pores that make them promising for applications in catalysis, but they typically suffer from poor stability. Now, adding methoxy groups to its pore walls has been shown to strengthen a COF's interlayer interactions, resulting in a stable, crystalline, porous material that can be further converted into chiral organocatalysts. |
 |
 |
 |
O-GlcNAc modification blocks the aggregation and toxicity of the protein α-synuclein associated with Parkinson's disease pp913 - 920
Nicholas P. Marotta, Yu Hsuan Lin, Yuka E. Lewis, Mark R. Ambroso, Balyn W. Zaro et al.
doi:10.1038/nchem.2361

O-linked N-acetyl-glucosamine (O-GlcNAc) has been identified as an endogenous modification of α-synuclein; however, its effect on the properties of the protein is unclear. Now, recombinant protein and synthetic peptides have been combined to produce both unmodified and site-specifically O-GlcNAc-modified a-synuclein. The O-GlcNAc modification at threonine 72 was shown to inhibit the aggregation and associated toxicity of α-synuclein. |
 |
 |
 |
Molecular hydrogen interacts more strongly when rotationally excited at low temperatures leading to faster reactions pp921 - 926
Yuval Shagam, Ayelet Klein, Wojciech Skomorowski, Renjie Yun, Vitali Averbukh et al.
doi:10.1038/nchem.2359

The rotational state of a molecule is not generally considered to play a role in how fast it reacts; however, when the temperature is low quantum effects become more important. Now, it is shown that at low temperatures rotationally excited H2 molecules react with He faster than non-rotating ground-state molecules — a process mediated by stronger long-range attraction. |
 |
 |
 |
Cage connectivity and frontier π orbitals govern the relative stability of charged fullerene isomers pp927 - 934
Yang Wang, Sergio Díaz-Tendero, Manuel Alcamí and Fernando Martín
doi:10.1038/nchem.2363

The stability of charged fullerenes is not as well understood as that of their neutral counterparts, with, for example, more frequent violations of the isolated-pentagon and pentagon-adjacency penalty rules. Now, a simple model based on the concepts of cage connectivity and frontier π orbitals predicts the relative stability of cationic and anionic fullerene isomers.
See also: News and Views by Fowler |
 |
 |
 |
Rhodium-catalysed asymmetric allylic arylation of racemic halides with arylboronic acids pp935 - 939
Mireia Sidera and Stephen P. Fletcher
doi:10.1038/nchem.2360
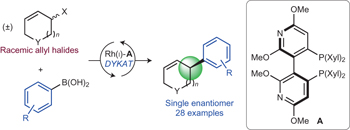
Cross-couplings between boronic acids and halides are a mainstay of synthetic organic chemistry but enantioselective Csp2–Csp3 couplings are rare, and simply retaining the stereochemistry of the starting material is problematic. Now, it is shown that racemic allylic halides can converted to single enantiomer products by a rhodium(I)-catalysed asymmetric allylic arylation using arylboronic acids
Chemical compounds |
 |
In Your Element |  Top Top |
 |
 |
 |
Strontium's scarlet sparkles p940
Francois-Xavier Coudert
doi:10.1038/nchem.2376
From sugar beets to TV screens, François-Xavier Coudert explores the history, applications and perils of the Scottish element, strontium |
 |
 Top Top |
 |
 |
 |
| Advertisement |
 |
Horticulture Research is an Open Access, fully peer-reviewed journal that publishes the best and most interesting research focusing on all major horticultural crops from around the world.
Submit your manuscript |  | | |
 |
| |
 |  |  |  |  |  | Natureevents is a fully searchable, multi-disciplinary database designed to maximise exposure for events organisers. The contents of the Natureevents Directory are now live. The digital version is available here.
Find the latest scientific conferences, courses, meetings and symposia on natureevents.com. For event advertising opportunities across the Nature Publishing Group portfolio please contact natureevents@nature.com |  |  |  |  |  |
|
 |


No comments:
Post a Comment