TABLE OF CONTENTS
|
January 2014 Volume 6, Issue 1 |
 |  |  |
 |  Thesis Thesis
 Research Highlights Research Highlights
 Blogroll Blogroll
 News and Views News and Views
 Articles Articles
 Erratum Erratum
 In Your Element In Your Element | |
 |
|
 |
 |
| Advertisement |
 |
NatureJournals app for ipad
The NatureJournals app for iPad now boasts over 25 Nature-branded titles. Subscribe to any journal in the app for $35.99* and gain access to world class research on the move.
www.nature.com/content/app/anytitleoffer
*Apple exchange rates apply. Limited time offer available on all journals except Scientific Reports. iPad is a trademark of Apple Inc. | | |
 |
| |
Thesis |  Top Top |
 |
 |
 |
Laughing matter pp1 - 2
Michelle Francl
doi:10.1038/nchem.1827
Michelle Francl takes a serious look at whether we should indulge in scientific humour. |
 |
Research Highlights |  Top Top |
 |
 |
 |
Sandwich complexes: Metals in the middle | Amorphous solids: Cage calculations | Photocycloadditions: Asymmetric at last | Nanoreactors: Catalysis in compartments |
Blogroll |  Top Top |
 |
 |
 |
Blogroll: New thinking p5
Joshua Howgego
doi:10.1038/nchem.1824 |
 |
News and Views |  Top Top |
 |
 |
 |
 Molecular dynamics: A stitch in time pp7 - 8
Xavier Deupi
doi:10.1038/nchem.1832
Lengthy molecular dynamics simulations of complex systems at the atomic scale usually require supercomputers. Now, by stitching together many shorter independent simulations run 'in the cloud', this requirement has been circumvented, allowing two milliseconds of the dynamics of a G-protein-coupled receptor to be simulated.
See also: Article by Kohlhoff et al. |  |  |  | Single-molecule chemistry: Knowing your neighbours pp8 - 10
Peter Liljeroth
doi:10.1038/nchem.1823
Quantitatively studying how the rate of a chemical reaction is affected by a reactant's atomic-scale environment is extremely challenging. This has now been achieved at the single-molecule level using scanning tunnelling microscopy to monitor tautomerization in an atomically well-defined environment.
See also: Article by Kumagai et al. |  |  |  | Biosynthesis: Non-stick natural products pp10 - 12
Peter A. Jordan and Bradley S. Moore
doi:10.1038/nchem.1828
Fluorine imparts many drugs with beneficial properties, however, the synthesis of fluorinated complex natural products is challenging. Biosynthetic strategies and recent experimental precedents have paved the way for bioengineered fluorinated polyketides. |  |  |  | Separated tandem catalysis: It's about time pp12 - 13
Sarah Abou-Shehada and Jonathan M. J. Williams
doi:10.1038/nchem.1826
One-pot processes in which a single catalyst carries out several reactions are attractive, but typically promote the formation of by-products as well as the desired ones, and are not amenable to optimization of the individual transformations. Now, these issues have been overcome by separating the catalytic processes in time.
See also: Article by Li & Herzon |  |  |  | Biophysical chemistry: Strength in numbers pp13 - 14
Graeme A. King and Gijs J. L. Wuite
doi:10.1038/nchem.1831
Replication of the HIV-1 viral genome can be inhibited by a protein known as APOBEC3G, via two seemingly contradictory mechanisms. Now, the molecular conundrum behind these two processes has been resolved.
See also: Article by Chaurasiya et al. |  | |  | | |
 |
Articles |  Top Top |
 |
 |
 |
Cloud-based simulations on Google Exacycle reveal ligand modulation of GPCR activation pathways pp15 - 21
Kai J. Kohlhoff, Diwakar Shukla, Morgan Lawrenz, Gregory R. Bowman, David E. Konerding et al.
doi:10.1038/nchem.1821

Two milliseconds of molecular dynamics simulations of a major drug-target G-protein-coupled receptor (GPCR) has been carried out using Google's Exacycle cloud computing platform. Markov state models were used to aggregate independent simulations into a statistical model that provides an atomistic description of GPCR ligand-modulated activation pathways.
Chemical compounds
See also: News and Views by Deupi |
 |
 |
 |
Temporal separation of catalytic activities allows anti-Markovnikov reductive functionalization of terminal alkynes pp22 - 27
Le Li and Seth B. Herzon
doi:10.1038/nchem.1799
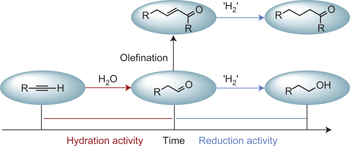
Multifunctional catalysts typically process substrates and intermediates concurrently. Here, a strategy is described to separate catalytic activities in the time domain (temporal separation). Application of this strategy has led to the development of a method to effect the anti-Markovnikov reductive functionalization of terminal alkynes; such an approach may facilitate the development of other synthetic reaction cascades.
Chemical compounds
See also: News and Views by Abou-Shehada & Williams |
 |
 |
 |
Oligomerization transforms human APOBEC3G from an efficient enzyme to a slowly dissociating nucleic acid-binding protein pp28 - 33
Kathy R. Chaurasiya, Micah J. McCauley, Wei Wang, Dominic F. Qualley, Tiyun Wu et al.
doi:10.1038/nchem.1795

HIV-1 replication is inhibited by the enzyme APOBEC3G via two separate mechanisms. A deamination mechanism requires rapid binding and release of single-stranded DNA (ssDNA), whereas a roadblock mechanism requires slow binding. Now APOBEC3G has been shown to initially bind ssDNA with rapid on–off rates. The enzyme subsequently converts via oligomerization to a slowly dissociating binding mode, which, it is proposed, inhibits reverse transcription.
See also: News and Views by King & Wuite |
 |
 |
 |
The aromatic ene reaction pp34 - 40
Dawen Niu and Thomas R. Hoye
doi:10.1038/nchem.1797
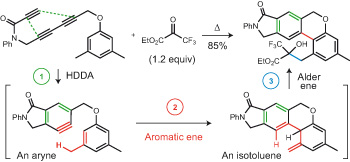
An aryne intermediate produced by a hexadehydro-Diels–Alder reaction is shown to engage in an unusual — and before this work rare and inefficient — aromatic ene reaction to produce an isotoluene. This second reactive intermediate can then engage an external enophile resulting in a conjunctive cascade in which four C–C bonds and three rings are created without involvement of reagents or generation of by-products.
Chemical compounds |
 |
 |
 |
Controlling intramolecular hydrogen transfer in a porphycene molecule with single atoms or molecules located nearby pp41 - 46
Takashi Kumagai, Felix Hanke, Sylwester Gawinkowski, John Sharp, Konstantinos Kotsis et al.
doi:10.1038/nchem.1804

The rate of an intramolecular hydrogen transfer reaction in a single porphycene molecule resting on a copper surface can be controlled by placing a copper adatom close to it. Cooperativity effects are also observed in rows of porphycene molecules, where the reaction rate of each individual molecule depends on the precise tautomer state of its neighbours.
See also: News and Views by Liljeroth |
 |
 |
 |
Ligand-enabled multiple absolute stereocontrol in metal-catalysed cycloaddition for construction of contiguous all-carbon quaternary stereocentres pp47 - 51
Kohsuke Ohmatsu, Naomichi Imagawa and Takashi Ooi
doi:10.1038/nchem.1796

A palladium-catalysed, highly enantio- and diastereoselective [3 + 2] cycloaddition reaction to produce densely functionalized pyrrolidine frameworks is developed by exploiting a new phosphine ligand with a chiral ammonium salt component. Rigorous control of the individual absolute stereochemistry of three contiguous stereocentres, including vicinal all-carbon quaternary stereocentres, is possible using this methodology.
Chemical compounds |
 |
 |
 |
Calculations predict a stable molecular crystal of N8 pp52 - 56
Barak Hirshberg, R. Benny Gerber and Anna I. Krylov
doi:10.1038/nchem.1818

Polynitrogen compounds are of interest on a fundamental level and as potential high-energy-density materials. A crystalline solid that consists of two isomeric forms of N8 molecules held together by weak van der Waals interactions has now been predicted to exist, and to be stable even at low pressures. |
 |
 |
 |
Biogenetically inspired synthesis and skeletal diversification of indole alkaloids pp57 - 64
Haruki Mizoguchi, Hideaki Oikawa and Hiroki Oguri
doi:10.1038/nchem.1798

Emulating the biogenesis of natural products, a synthetic strategy is described in which an achiral multipotent intermediate reacts through three distinct [4 + 2] cyclizations and two types of redox-mediated annulation. This results in divergent access to natural product-like scaffolds in 6–9 steps. The efficiency of this approach is highlighted in the total syntheses of three natural products.
Chemical compounds |
 |
 |
 |
Discrimination of epimeric glycans and glycopeptides using IM-MS and its potential for carbohydrate sequencing pp65 - 74
P. Both, A. P. Green, C. J. Gray, R. ?ardzík, J. Voglmeir et al.
doi:10.1038/nchem.1817

Identification of glycosylation patterns is complicated by the lack of sensitive analytical techniques that can distinguish between epimeric carbohydrates. It has now been shown that ion-mobility tandem mass spectrometry of ions derived from glycopeptides and oligosaccharides enables glycan stereochemistry to be determined, highlighting the potential of this technique for sequencing complex carbohydrates on cell surfaces. |
 |
 |
 |
Visualization and selective chemical targeting of RNA G-quadruplex structures in the cytoplasm of human cells pp75 - 80
Giulia Biffi, Marco Di Antonio, David Tannahill and Shankar Balasubramanian
doi:10.1038/nchem.1805

The presence of RNA G-quadruplex structures in human cells using a structure-specific antibody is demonstrated. Using small molecules, the selective stabilization of cytoplasmic RNA G-quadruplexes versus nuclear DNA G-quadruplexes is demonstrated. These findings validate the existence of RNA G-quadruplexes and their specific targeting by small molecules within a cellular context.
Chemical compounds |
 |
Erratum |  Top Top |
 |
 |
 |
Erratum: Singlet exciton fission in solution p81
Brian J. Walker, Andrew J. Musser, David Beljonne and Richard H. Friend
doi:10.1038/nchem.1829 |
 |
In Your Element |  Top Top |
 |
 |
 |
Argon out of thin air p82
Markku Räsänen
doi:10.1038/nchem.1825
Markku Rasanen remembers making a neutral compound featuring argon, and ponders on the reactivity of this inert element. |
 |
 Top Top |
 |
 |
 |
| Advertisement |
 |
| |
 |
| |
 |  |  |  |  |  | Natureevents is a fully searchable, multi-disciplinary database designed to maximise exposure for events organisers. The contents of the Natureevents Directory are now live. The digital version is available here.
Find the latest scientific conferences, courses, meetings and symposia on natureevents.com. For event advertising opportunities across the Nature Publishing Group portfolio please contact natureevents@nature.com |  |  |  |  |  | |
 |


No comments:
Post a Comment