TABLE OF CONTENTS
| September 2012 Volume 8, Issue 9 |  |  |  |  |  Research Highlights Research Highlights
 News and Views News and Views
 Brief Communications Brief Communications
 Articles Articles
| |  | |  |  | | Advertisement |  | The Open Innovation Pavilion has a fresh set of Challenges that need a winning solution from biologists like you. Submit a proposed solution for any of our open Challenges for a chance to win up to $1 million!
View all open Challenges by clicking here.
Click here to see stories from past Solvers. | |  | | | Research Highlights |  Top Top |  |  |  | Regulation: Positively alarming | Virology: Cholesterol manipulation | Proteomics: Cross-talking modifications | Synthetic biology: Beta testing | Drug resistance: The stroma's contribution | Iron-sulfur clusters: MMS to the nucleus | Prodrug activation: Glutaredoxin family tree | Immunity: FetA gets fat | News and Views |  Top Top |  |  |  | |  | Brief Communications |  Top Top |  |  |  | |  |  |  | Expanding the genetic code of Drosophila melanogaster pp748 - 750
Ambra Bianco, Fiona M Townsley, Sebastian Greiss, Kathrin Lang and Jason W Chin
doi:10.1038/nchembio.1043
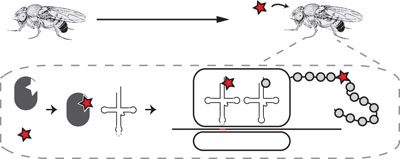
Genetic code expansion by ribosomal incorporation of non-natural amino acids has provided a useful approach for site-specific protein modification. This approach has now been extended to the model organism Drosophila melanogaster, permitting the introduction of non-standard amino acids into proteins within specific cell and tissue types and across developmental stages.
Chemical compounds |
|
|  | Articles |  Top Top |  |  |  | AID/APOBEC deaminases disfavor modified cytosines implicated in DNA demethylation pp751 - 758
Christopher S Nabel, Huijue Jia, Yu Ye, Li Shen, Hana L Goldschmidt, James T Stivers, Yi Zhang and Rahul M Kohli
doi:10.1038/nchembio.1042
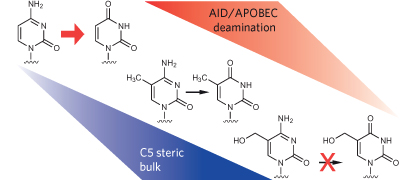
AID/APOBEC deaminases, which convert cytosine bases to uracils in DNA and RNA, have recently been assigned a role in epigenetic regulation as components of DNA demethylation pathways. A systematic study shows that AID/APOBEC enzymes preferentially deaminate unmodified cytosine over its C5-modified forms, calling into question the plausibility of deaminase-mediated DNA demethylation pathways.
|
|
|  |  |  | |  |  |  | Discovery of glycosyltransferases using carbohydrate arrays and mass spectrometry pp769 - 773
Lan Ban, Nicholas Pettit, Lei Li, Andreea D Stuparu, Li Cai, Wenlan Chen, Wanyi Guan, Weiqing Han, Peng George Wang and Milan Mrksich
doi:10.1038/nchembio.1022
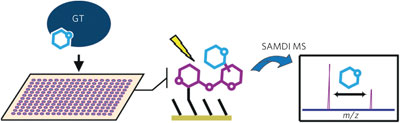
Discovery of the native activity of the ~60,000 putative glycosyltransferases remains a substantial challenge. A high-throughput, label-free method drastically speeds this process, with assays of 85 enzymes, 24 acceptors and 7 donors returning functions for four new proteins.
Chemical compounds
See also: News and Views by Gildersleeve |
|
|  |  |  | |  |  |  | |  |  |  | A stand-alone adenylation domain forms amide bonds in streptothricin biosynthesis pp791 - 797
Chitose Maruyama, Junya Toyoda, Yasuo Kato, Miho Izumikawa, Motoki Takagi, Kazuo Shin-ya, Hajime Katano, Takashi Utagawa and Yoshimitsu Hamano
doi:10.1038/nchembio.1040
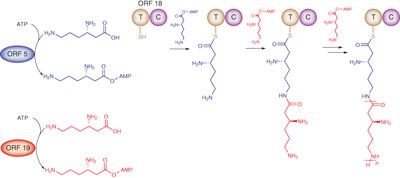
A study of three synthetases involved in streptothricin biosynthesis demonstrates roles for two A domains in activating lysine, with one A domain transferring lysine to a carrier T domain and the second directly catalyzing amide bond formation to form a growing lysine oligopeptide.
Chemical compounds |
|
|  |  |  | A role for the root cap in root branching revealed by the non-auxin probe naxillin pp798 - 805
Bert De Rybel, Dominique Audenaert, Wei Xuan, Paul Overvoorde, Lucia C Strader, Stefan Kepinski, Rebecca Hoye, Ronald Brisbois, Boris Parizot, Steffen Vanneste, Xing Liu, Alison Gilday, Ian A Graham, Long Nguyen, Leentje Jansen, Maria Fransiska Njo, Dirk Inzé, Bonnie Bartel and Tom Beeckman
doi:10.1038/nchembio.1044
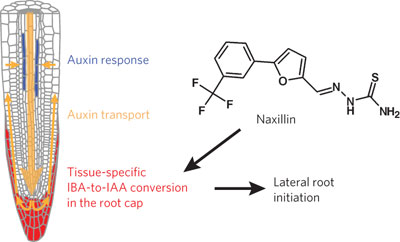
The plant hormone auxin affects many aspects of root development, including lateral root branching. A high-throughput screen in Arabidopsis thaliana has led to the identification of naxillin, a non-auxin chemical probe that enhances lateral root branching and has revealed an important role of the root cap in regulating this process.
Chemical compounds |
|
|  |  Top Top |  |  | | Advertisement |  | 
Online-only personal subscriptions now available
to Nature Chemistry and Nature Chemical Biology
For only 49 USD/29 GBP/29 EUR
Subscribe now! | |  | | |  |  |  |  |  |  | Natureevents is a fully searchable, multi-disciplinary database designed to maximise exposure for events organisers. The contents of the Natureevents Directory are now live. The digital version is available here.
Find the latest scientific conferences, courses, meetings and symposia on natureevents.com. For event advertising opportunities across the Nature Publishing Group portfolio please contact natureevents@nature.com |  |  |  |  |  |
|  | |
|


No comments:
Post a Comment