TABLE OF CONTENTS
| February 2012 Volume 8, Issue 2 |  |  |  |  |  Poster Poster
 Research Highlights Research Highlights
 News and Views News and Views
 Brief Communications Brief Communications
 Articles Articles
| |  | |  |  | | Advertisement |  | Nature Chemical Biology
Free Poster on Human protein methyltransferases
Nature Chemical Biology presents a poster highlighting the human protein methyltransferase families, the small molecules known to target them and the prospects for PMT-focused drug development.
Download the Poster today by visiting:
www.nature.com/nchembio/poster/hpm
Poster sponsored by:
 | |  | | | Poster |  Top Top |  |  |  | Human protein methyltransferases
 The poster "The human protein methyltransferases," which highlights the families of protein methyltransferases (PMTs) and explores prospects for PMT-focused drug development, accompanies this issue. The poster was produced with support from Epizyme. The poster "The human protein methyltransferases," which highlights the families of protein methyltransferases (PMTs) and explores prospects for PMT-focused drug development, accompanies this issue. The poster was produced with support from Epizyme.
|  | Research Highlights |  Top Top |  |  |  | Protein-protein interactions: In the TANK | Enzyme engineering: Just a jump to the left | Methods: Zebrafish click | Allostery: A systematic search | Metabolism: Closing the loop | Translation: Ribosome 3.0 | Molecular switches: The pore of the matter | Chemical genetics: Bumping into AMPK substrates | News and Views |  Top Top |  |  |  | |  | Brief Communications |  Top Top |  |  |  | Skepinone-L is a selective p38 mitogen-activated protein kinase inhibitor pp141 - 143
Solveigh C Koeberle, Johannes Romir, Stefan Fischer, Andreas Koeberle, Verena Schattel, Wolfgang Albrecht, Christian Grütter, Oliver Werz, Daniel Rauh, Thilo Stehle and Stefan A Laufer
doi:10.1038/nchembio.761
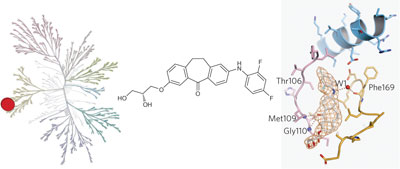
Studies elucidating the role of p38α MAPK in diverse biological processes are limited by the lack of a specific inhibitor. Skepinone-L is a highly selective ATP-competitive inhibitor of p38 with excellent potency in vivo.
First paragraph | Full Text | PDF | Chemical compounds |
|
|  |  |  | Chemical informatics and target identification in a zebrafish phenotypic screen pp144 - 146
Christian Laggner, David Kokel, Vincent Setola, Alexandra Tolia, Henry Lin, John J Irwin, Michael J Keiser, Chung Yan J Cheung, Daniel L Minor Jr, Bryan L Roth, Randall T Peterson and Brian K Shoichet
doi:10.1038/nchembio.732
A cheminformatics approach helps identify proteins such as GPCRs, ion channels and kinases that are targeted by small molecules that give phenotypes in a zebrafish behavioral assay.
First paragraph | Full Text | PDF | Chemical compounds |
|
|  | Articles |  Top Top |  |  |  | Knot formation in newly translated proteins is spontaneous and accelerated by chaperonins pp147 - 153
Anna L Mallam and Sophie E Jackson
doi:10.1038/nchembio.742
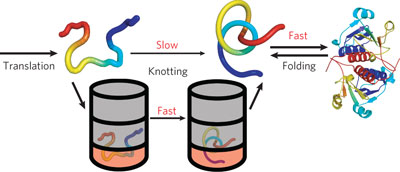
Threading of a polypeptide chain is required for knotted proteins to adopt active conformations. A cell-free translation system in conjunction with pulse proteolysis to track folding of trefoil knotted proteins reveal that these knots form spontaneously, but GroEL–GroES enhances the rate of post-translational knot formation.
Abstract | Full Text | PDF |
|
|  |  |  | A ketosynthase homolog uses malonyl units to form esters in cervimycin biosynthesis pp154 - 161
Tom Bretschneider, Georg Zocher, Michelle Unger, Kirstin Scherlach, Thilo Stehle and Christian Hertweck
doi:10.1038/nchembio.746
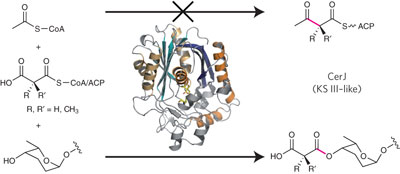
Polyketide synthases make use of a limited set of enzymes to create diverse natural products. The discovery that a ketosynthase homolog catalyzes ester bond formation instead of the typical Claisen condensation uncovers a previously unknown mechanism to generate chemical diversity.
Abstract | Full Text | PDF | Chemical compounds |
|
|  |  |  | How subunits cooperate in cAMP-induced activation of homotetrameric HCN2 channels pp162 - 169
Jana Kusch, Susanne Thon, Eckhard Schulz, Christoph Biskup, Vasilica Nache, Thomas Zimmer, Reinhard Seifert, Frank Schwede and Klaus Benndorf
doi:10.1038/nchembio.747
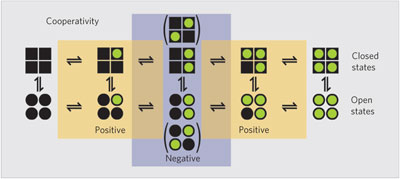
The four subunits of the tetrameric voltage-sensitive HCN channel have different cAMP binding affinities, with the second and fourth binding events being positively cooperative and the third being negatively cooperative, suggesting a double-dimeric organization of the channel.
Abstract | Full Text | PDF
See also: News and Views by Zhou |
|
|  |  |  | Primary amines protect against retinal degeneration in mouse models of retinopathies pp170 - 178
Akiko Maeda, Marcin Golczak, Yu Chen, Kiichiro Okano, Hideo Kohno, Satomi Shiose, Kaede Ishikawa, William Harte, Grazyna Palczewska, Tadao Maeda and Krzysztof Palczewski
doi:10.1038/nchembio.759
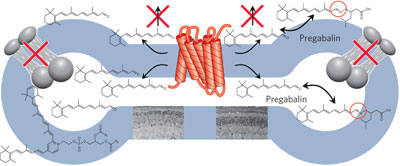
Compounds containing a primary amino group can protect against light-induced retinal degeneration in a mouse model of Stargardt’s disease and age-related retinal degeneration by forming a transient Schiff base with all-trans-retinal, lowering its intracellular concentration and preventing accumulation of lipofuscin chromophores in the eye.
Abstract | Full Text | PDF |
|
|  |  |  | Natural product–inspired cascade synthesis yields modulators of centrosome integrity pp179 - 184
Heiko Dückert, Verena Pries, Vivek Khedkar, Sascha Menninger, Hanna Bruss, Alexander W Bird, Zoltan Maliga, Andreas Brockmeyer, Petra Janning, Anthony Hyman, Stefan Grimme, Markus Schürmann, Hans Preut, Katja Hübel, Slava Ziegler, Kamal Kumar and Herbert Waldmann
doi:10.1038/nchembio.758
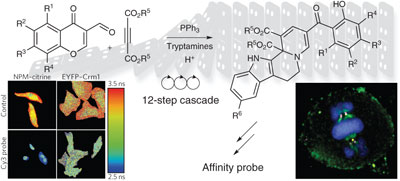
Natural product–inspired compounds are primed to interact with and manipulate biological processes, but obtaining these complex molecules poses synthetic challenges. The development of a 12-step, 1-pot cascade reaction leads to the 'centrocountins', tetrahydroindoloquinolizines that modulate mitosis by targeting the centrosome-associated proteins nucleophosmin and Crm1.
Abstract | Full Text | PDF | Chemical compounds |
|
|  |  |  | Small-molecule proteostasis regulators for protein conformational diseases pp185 - 196
Barbara Calamini, Maria Catarina Silva, Franck Madoux, Darren M Hutt, Shilpi Khanna, Monica A Chalfant, S Adrian Saldanha, Peter Hodder, Bradley D Tait, Dan Garza, William E Balch and Richard I Morimoto
doi:10.1038/nchembio.763
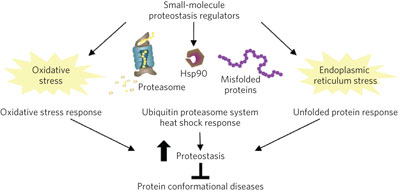
Screens of large compound libraries identify new small–molecule proteostasis regulators that, by enhancing the activity of the heat shock response factor HSF–1 and by activating other components of the proteostasis network, such as the antioxidant response or the unfolded protein response pathways, restore protein folding in multiple models of protein conformational diseases.
Abstract | Full Text | PDF |
|
|  |  |  | Disease-specific non–reducing end carbohydrate biomarkers for mucopolysaccharidoses pp197 - 204
Roger Lawrence, Jillian R Brown, Kanar Al-Mafraji, William C Lamanna, James R Beitel, Geert-Jan Boons, Jeffrey D Esko and Brett E Crawford
doi:10.1038/nchembio.766
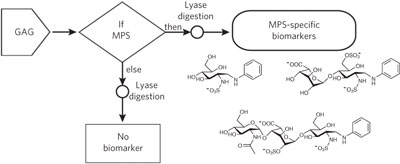
Mucopolysaccharidoses are caused by a deficiency in GAG processing and can often be treated if the dysfunctional enzyme can be identified. A chemical derivatization strategy in combination with biochemical logic now yields a series of biomarkers that can be used to distinguish these disorders.
Abstract | Full Text | PDF | Chemical compounds
See also: News and Views by Kjellen |
|
|  |  |  | Timing facilitated site transfer of an enzyme on DNA pp205 - 210
Joseph D Schonhoft and James T Stivers
doi:10.1038/nchembio.764
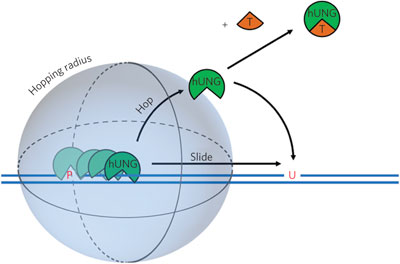
DNA repair proteins require efficient pathways to search for DNA damage lesions within a large genome. Kinetic trapping experiments define the rates of human uracil DNA glycosylase hopping and sliding along damaged DNA substrates and highlight the role of the phosphodiester backbone in DNA sliding.
Abstract | Full Text | PDF |
|
|  |  |  | Oxysterols are allosteric activators of the oncoprotein Smoothened pp211 - 220
Sigrid Nachtergaele, Laurel K Mydock, Kathiresan Krishnan, Jayan Rammohan, Paul H Schlesinger, Douglas F Covey and Rajat Rohatgi
doi:10.1038/nchembio.765
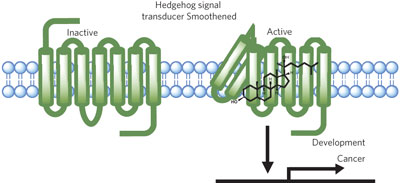
20(S)-hydroxycholesterol binds to Smo at a site distinct from cyclopamine and activates signaling via an allosteric mechanism. A new alkyne oxysterol analog selectively captures Smo from membrane extracts and provides a new method for detecting protein-sterol interactions.
Abstract | Full Text | PDF | Chemical compounds
See also: News and Views by Sharpe & de Sauvage |
|
|  |  Top Top |  |  | | Advertisement |  | |  | | |  |  |  |  |  |  | Natureevents is a fully searchable, multi-disciplinary database designed to maximise exposure for events organisers. The contents of the Natureevents Directory are now live. The digital version is available here.
Find the latest scientific conferences, courses, meetings and symposia on natureevents.com. For event advertising opportunities across the Nature Publishing Group portfolio please contact natureevents@nature.com |  |  |  |  |  |
|  | |
|


No comments:
Post a Comment